Tue 29 Nov 2005
Cytotoxicity of Vibrio parahaemolyticus Thermostable Direct Hemolysin on cultured Rat-1 Cells Is Not Due to Colloidal Osmotic Effect
Posted by rochman under Jvet Vol 6(2) 2005(SITOTOXISITAS DARI VIBRIO PARAHAEMOLYTICUS THERMOSTABLE DIRECT HEMOLYSIN (TDH) PADA KULTUR SEL BUKAN KARENA EFEK KOLOIDAL OSMOTIK)
ROCHMAN NAIM
Faculty of Veterinary Medicine – Bogor Agricultural University
Jalan Agatis Kampus IPB Darmaga, BOGOR 16680
Tel/Fax:0251-625959, e-mail: [email protected]
ABSTRACT
Vibrio parahaemolyticus thermostable direct hemolysin (TDH) has previously been reported to cause osmotic lysis on erythrocytes. This study was aimed to investigate whether TDH has a colloidal osmotic effect on cultured cells, Rat-1 cells were exposed to TDH in the presence of osmotic stabilizers. The presence of osmotic stabilizers such as PEG and dextran could not inhibit the cytotoxicity of TDH. Neither PEG nor dextran could reduce TDH-induced morphological changes. These findings suggest that TDH cytotoxicity on Rat-1 cells is not due to colloidal osmotic effect.
Key words: Vibrio parahaemolyticus, Cytotoxicity, Hemolysin
ABSTRAK
Vibrio parahaemolyticus thermostable direct hemolysin (TDH) telah dilaporkan menyebabkan lisis osmotik pada eritrosit. Studi ini bertujuan untuk mengetahui lebih lanjut apakah TDH memiliki efek osmotik kolloidal pada kultur sel. Sel Rat-1 diekspos ke TDH dengan keberadaan osmotic stabilizer. Keberadaan osmotic stabilizer seperti PEG dan dekstran tidak dapat menghambat sitotoksisitas TDH. PEG dan dekstran tidak dapat mengurangi perubahan morfologik yang diakibatkan TDH. Temuan ini menunjukkan bahwa sitotoksisitas TDH pada sel Rat-1 tidak disebabkan oleh efek osmotik kolloidal.
Kata-kata kunci: Vibrio parahaemolyticus, sitotoksisitas, Hemolisin
INTRODUCTION
Vibrio parahaemolyticus is a major cause of gastroenteritis in areas of the world where seafood is a major part of diet (Janda et al., 1988; Joseph et al., 1982). Thermostable direct hemolysin (TDH) is considered as an important virulence factor in V. parahaemolyticus gastroenteritis (Honda and Iida, 1993). A number of biological properties have been attributed to TDH including hemolytic activity, enterotoxicity, cytotoxicity, and cardiotoxicity (Honda and Iida, 1993).
The cytotoxic effects of TDH have been observed with cultured cells. After exposure to TDH, morphological damage occurs in cultured mouse myocardial and mouse melanoma cells (Goshima et al., 1978). Similarly, TDH causes morphological changes in the microvilli, degradation of the cytoplasm of the cells, and complete disintegration of the nuclei in FL cells (Sakurai et al., 1976). In erythrocytes, TDH has been reported to cause colloid osmotic lysis (Honda et al., 1992). Although a number of studies dealing with TDH have been reported, its mechanism of action on cultured cells still remains unclear.
In this study, the effects of osmotic stabilizers on the cytotoxicity of TDH on cultured rat embryonic fibroblast cells (Rat-1) which are sensitive to TDH were investigated.
MATERIALS AND METHODS
Cell culture
Rat-1 cells were obtained from the American Type Culture Collection. These cells were grown in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum and 100 g/ml gentamicin. The cells were incubated at 37oC in an atmosphere of 5% CO2-95% air.
Preparation of purified TDH
Plasmid vector pTI101 harboring the structural gene of wild type TDH (Iida et al., 1995) was introduced into Escherichia coli JM109 by transformation. The transformants were cultivated in Luria-Bertani broth (consisting of 1% Bacto Tryptone, 0.5% Bacto yeast extract, and 0.5% NaCl) containing 100 g/ml ampicillin at 37oC for 16 h with shaking. After incubating, the culture filtrate was collected by centrifugation. Synthesized TDH was purified by a previously described method (Honda et al., 1976). Briefly, crude toxin was isolated by addition of ammonium sulfate (35.1 grams per 100 ml) to culture filtrate. This crude toxin was subjected to a series of column chromatography including diethylaminoethyl cellulose, hydroxyapatite and Sephadex G-75 columns. Fractions containing TDH were assayed by hemolysis on a rabbit blood agar plate. The purity of samples was examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis according to the method of Laemmli (Laemmli, 1970). Protein concentration of the purified TDH was determined using the method of Lowry (Lowry et al., 1951).
Viability assay
Cells prepared in a 96 well plates (approximately 104 cells/well) were exposed to TDH for 30 min at 37oC in an atmosphere of 5% CO2-95% air in 100 l DMEM in the presence or absence of osmotic stabilizers. The final con-centrations for glucose, raffinose, PEG and dextran were 100 mM, 100 mM, 15 mM and 10 mM, respectively, because of their solubility. Cell viability was assessed by a Cell Counting Kit (Dojindo, Japan) which employs water soluble tetrazolium salt reduced by dehydrogenase into formazan. As positive controls, cells were treated with 1% Triton X-100 and negative controls were those without toxin treatment. Optical density was measured on the Titertek Multiskan MCC/340 MKII (Lab-systems, Finland) at 450 nm. Viability was calculated using the optical density of toxin treated (ODt), positive control (ODpc), and negative control (ODnc) samples. The viability formula was (ODt – ODpc)/(ODt – ODnc) × 100%.
Morphological studies
Cells were grown onto glass coverslips in 6-well plates until approximately 90% confluent. After being seeded, cells were exposed to TDH in DMEM in the absence or presence of PEG or dextran for 30 min at 37oC. As a control, cells were not treated with TDH. After the treatments, both control and toxin-treated cells were fixed and stained with Giemsa, and examined at a magnification of ×400 with standard bright-field light microscopy.
Giemsa staining
Samples were fixed in absolute methanol for 5 minutes and then air dried at room temperature for 10 minutes. After drying, samples were stained with Giemsa stain for 10 minutes and washed with distilled water. Samples were then dehydrated with ethanol or isopropyl alcohol and cleared with xylene (xylol).
RESULTS
TDH has previously been reported to cause colloidal osmotic hemolysis on erythrocytes (Honda et al., 1992). Using PEG (15 mM) and dextran (10 mM), I confirmed that such osmotic stabilizers can protect rabbit erythrocytes against TDH-induced hemolysis. To further investigate whether TDH has a colloidal osmotic effect on cultured cells, Rat-1 cells were exposed for 30 min to TDH in the presence or absence of osmotic stabilizers. The cytotoxicity of TDH was dose-dependent in the absence of osmotic stabilizers (Fig. 1). The presence of glucose (0.72 nm in diameter), raffinose (1.14 nm) PEG (3.80 nm) and dextran (4.60 nm) could not inhibit the cytotoxicity of TDH (Fig. 1).
Figure 1. Viability of Rat-1 cells exposed to TDH in the presence or absence of
osmotic stabilizer.
Cells were exposed for 30 min to TDH with or without osmotic stabilizers int DMEM. The viability of cells were determined by a Cell Counting Kit. Data show mean and standard deviation values from three independent experiments.
I visually assessed the effects of TDH on the cells in the presence or absence of PEG and dextran after 30 min of exposure. Untreated control Rat-1 cells had cells of uniform size (Fig. 2A). Rat-1 cells exposed to TDH showed morphological changes including detachment of cells from their neighbors, apparent loss of cell cytoplasm with shrinkage of most of the cells and reduction in the size of nuclei (Fig. 2A). Neither PEG (15 mM) nor dextran (10 mM) could reduce the morphological changes induced by TDH on Rat-1 cells (Figs. 2B and 2C). These findings suggest that TDH cytotoxixity on Rat-1 cells is not due to a colloidal osmotic effect.
Cells were exposed for 30 min to TDH with or without the osmotic stabilizers, PEG and dextran, in DMEM. For control, cells were not exposed to TDH. Samples were fixed and stained with Giemsa. Cells were incubated without osmotic stabilizers (A), with PEG (B), and with dextran (C). Magnification is×400
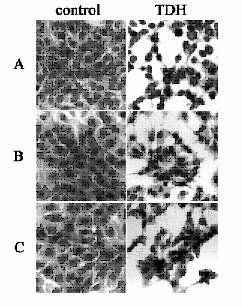
Figure 2. Morphology of Rat-1 cells exposed to TDH in the presence or absence
of osmotic stabilizers.
DISCUSSION
Mechanism of action of bacterial protein toxins can be categorized into three general groups: toxin acting at the plasma membrane level, toxins that bind to a receptor and stimulates the release of a second messenger, and toxins acting inside cells. The first one (e.g., Staphylococcus aureus alpha hemolysin) attacks target cells by increasing the membrane permeability to ions and small molecules. When the number of pores formed on a cell becomes relatively large, the intra- and extracellular concentrations of ions and other small solutes equilibrate thus creating an osmotic imbalance due to the asymmetric distribution of internal macromolecules. This causes an uncontrolled influx of extracellular ions, other small solutes, and water into the cell whose plasma membrane swells and eventually may be disrupted. Under these conditions, cell will undergo osmotic lysis, releasing their contents. The second one (e.g., Escherichia coli heat-stable enterotoxin a), toxins bind to their receptors on cell membrane and then stimulate the release of asecond messenger, most often with subsequent stimulation of a protein kinase. The last one (e.g., diphtheria toxin, Shiga toxin, cholera toxin) must be able to bind to their receptors and penetrate cells in oreder to perform their intracellular action. The process of cell intoxication by these toxin can be dissected into four different steps including cell binding, internalization, membrane translocation into the cytosol and modification of a selected cytosolic target (Bhakdi and Tranum-Jensen , 1991; Crane et al., 1992; Menestrina et al., 1994; Montecucco et al., 1994; Wern, 1992).
TDH has previously been considered to be a pore-forming toxin which causes colloidal osmotic hemolysis on erythrocytes (Honda et al., 1992). To investigate the effects of osmotic stabilizers on the cytotoxicity of TDH, Rat-1 cells were exposed to TDH in the presence or absence of osmotic stabilizers.
Osmotic stabilizers are agents which do not penetrate the plasma membrane of eukaryotic cells, but inhibit cytolytic toxins by decreasing colloidal osmotic swelling (McClane and McDonel, 1981). The ability of osmotic stabilizers to prevent cell swelling is proportional to their molecular weight and concentration in medium. I found that osmotic stabilizers did not protect Rat-1 cells against TDH cytotoxicity. Viability of TDH-exposed cells in the presence of osmotic stabilizers was not reduced. Furthermore, whether PEG or dextran were present or not made no difference to the TDH-induced morphological changes.
Previous studies have suggested that the action of TDH on red blood cells involves a disruption of the colloid osmotic equilibrium. Osmotic stabilizers were protective against TDH-induced hemolysis (Honda et al., 1992). In contrast, experiments reported here with osmotic stabilizers did not appear to be able to protect Rat-1 cells from the cytotoxicity of TDH. These results suggest that the mechanism of cytotoxicity of TDH on Rat-1 cells was different from that of hemolytic activity of TDH on red blood cells.
Acknowledgements
I am indebted to Prof. Honda and his staffs for having accepted and supporting me to perform this study in his laboratory.
REFERENCES
Bhakdi, S., and J. Tranum-Jensen. 1991. Alpha-toxin of Staphylococcus aureus. Microbiol Rev 55: 733-751.
Crane, J.K., M.S. Wehner, E.J. Bolen, J.J .Sando, J. Linden, R.L. Guerrant, and C.L. Sears. 1992. Regulation of intestinal guanylate cyclase by the heat-stable enterotoxin of Escherichia coli (STa) and protein kinase C. Infect Immun 60: 5004-5012.
Goshima, K., K. Owaribe, H. Yamanaka, and S. Yoshino. 1978. Requirements of calcium ions for cell degeneration with a toxin (vibriolysin) from Vibrio parahaemolyticus. Infect Immun 22: 821-832.
Honda, T., S. Taga, T. Takeda, M.A. Hasibuan, Y. Takeda, and T. Miwatani. 1976 Identification of lethal toxin with the thermostable direct hemolysin produced by Vibrio parahaemolyticus and some physicochemical properties of the purified toxin. Infect Immun 13: 133-139.
Honda, T., Y. Ni, T. Miwatani, T. Adachi, and J. Kim. 1992. The thermostable direct hemolysin of Vibrio parahaemolyticus is a pore-forming toxin. Can J Microbiol 38: 1175-1180.
Honda, T., and T. Iida. 1993. The pathogenicity of Vibrio parahaemolyticus and the role of the thermostable direct haemolysin and related haemolysins. Rev Med Microbiol 4: 106-113.
Iida, T., G. Tang, S. Suttikulpitug, K. Yamamoto, T. Miwatani, and T. Honda. 1995. Isolation of mutant toxins of Vibrio parahaemolyticus hemolysins by in vitro mutagenesis. Toxicon 33: 209-216.
Janda, J.M., C. Powers, R.G. Bryant, and S. Abbot. 1988. Current pers-pectives on the epidemiology and pathogenesis of clinically significant Vibrio spp. Clin Microbiol Rev 1: 245-267.
Joseph, S.W., R.R. Colwell, and J.B. Kaper. 1982. Vibrio parahaemolyticus and related halophilic vibrios. Crit Rev Microbiol 10: 77-124.
Laemmli, U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 227: 680-685.
Lowry, O.H., N.J. Rosebrough, A.L. Farr, and R.J. Randall. 1951. Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265-275.
McClane, B.A., and J.L. McDonel. 1981. Protective effects of osmotic stabilizers on morphological and permeability alterations induced in Vero cells by Clostridium perfringens enterotoxin. Biochim Biophys Acta 641: 401-409.
Menestrina, G., G. Schiavo, and C. Montecucco. 1994. Molecular mechanism of action of bacterial protein toxins. Molec Aspects Med 15: 79-193.
Montecucco, C., E. Papini, and G. Schiavo. 1994. Bacterial protein toxins penetrate cells via a four-step mechanism. FEBS Lett 346: 92-98.
Sakurai, J., T. Honda, Y. Jinguji, M. Arita, and T. Miwatani. 1976. Cytotoxic effect of the thermostable direct hemolysin produced by Vibrio parahaemolyticus on FL cells. Infect Immun 13: 873-876.
Wren, B.W. 1992. Bacterial enterotoxin interactions, In Molecular biology of bacterial infection: current status and future perspectives. Hormaeche, C.E., Penn, C.W., and Smyth, C.J. (eds.). Cambridge University Press. Cambridge. pp.127-147.