Thu 1 Dec 2005
The Ability Of Rat Cauda Epididymal Spermatozoa Cryopreserved Without Cryoprotectant In Liquid Nitrogen To Induce Pronucleus Formation
Posted by admin under Jvet Vol 6(3) 2005(KEMAMPUAN SPERMATOZOA CAUDA EPIDIDYMIS TIKUS YANG DIKRIOPRESERVASI TANPA KRIOPROTEKTAN DI DALAM NITROGEN CAIR GUNA MERANGSANG PEMBENTUKAN PRONUKLEUS)
Takdir Saili1 and Syahruddin Said2
1Faculty of Agriculture, Haluoleo University, Kampus Bumi Tri Dharma Anduonohu, Kendari 93232, 2Research Center for Biotechnology, Indonesian Institute of Sciences (LIPI), Jalan Raya Bogor Km 46, Cibinong
ABSTRACT
In the present study we have evaluated the ability of rat cauda epididymal spermatozoa cryopreserved without cryoprotectant in liquid nitrogen (-196C) to induce pronucleus formation by using ICSI method. The results showed that 45% and 64% oocytes were success ICSI when injected by freeze-thawed spermatozoa and fresh sperm, respectively, while the rests were fail to be activated after 10 hours of culture in modified Krebs-Ringer-Bicarbonate medium (mKRB) at 5% CO2 incubator, and 37C. By using lacmoid staining, it is revealed that among the success ICSI oocytes, 37% and 69% oocytes were activated when injected by freeze-thawed spermatozoa and fresh spermatozoa, respectively. Moreover, among activated oocytes injected by freeze-thawed spermatozoa showed 11% oocytes having two pronucleus, 8% oocytes having decondensed spermatozoa and 18% oocytes having condensed spermatozoa. Whereas 14% oocytes having condensed spermatozoa, 28% oocytes having decondensed spermatozoa and 27% oocytes having two pronucleus were gained when activated oocytes were injected by fresh spermatozoa. Based on the results it was concluded that injection of oocytes with rat cauda epididymal spermatozoa cryopreserved without cryoprotectant in liquid nitrogen could develop injected oocytes to formation of male and female pronucleus.
Keywords : ICSI, rat cauda epididymal spermatozoa, pronucleus.
ABSTRAK
Pada penelitian ini kami mengevaluasi kemampuan spermatozoa cauda epididymis yang dibekukan tanpa krioprotektan di dalam nitrogen cair (-196C) untuk mendukung pembentukan pronukleus jantan dan betina pada sel telur tikus melalui metode mikrofertilisasi ICSI. Hasil penelitian menunjukkan bahwa sejumlah 45% dan 64% sel telur mempunyai bentuk yang normal (sukses ICSI) setelah diinjeksi menggunakan masing-masing spermatozoa beku dan segar yang selanjutnya dikultur pada medium mKRB di dalam inkubator CO2 5%, 37C selama 10 jam. Melalui pewarnaan aceto lacmoid diperoleh hasil bahwa terdapat 37% dan 69% sel telur yang sukses ICSI berhasil teraktivasi setelah diinjeksi masing-masing dengan spermatozoa beku dan segar. Di antara sel telur yang teraktivasi setelah diinjeksi dengan spermatozoa beku, terdapat 11% sel telur memiliki dua pronukleus, 8% sel telur memilki decondensed spermatozoa dan 18% sel telur memiliki condensed spermatozoa. Sedangkan di antara sel telur yang teraktivasi setelah diinjeksi dengan spermatozoa segar terdapat 27% sel telur memiliki dua pronukleus, 28% sel telur memiliki decondensed spermatozoa dan 14% sel telur memiliki condensed spermatozoa. Berdasarkan hasil yang diperoleh dapat disimpulkan bahwa injeksi sel telur menggunakan spermatozoa cauda epididymis yang dikriopreservasi tanpa krioprotektan di dalam nitrogen cair dapat mendukung pembentukan pronukleus jantan dan betina pada tikus.
Kata kunci : ICSI, spermatozoa cauda epididymis tikus, pronukleus
INTRODUCTION
Development of various techniques for assisted reproduction including in vitro maturation (IVM) of oocytes, in vitro fertilization (IVF) of oocytes and in vitro culture (IVC) of embryos could play an important role in managing captive and natural populations of animals as well as in sustaining both genetic and global biodiversity (Wildt et al., 1992). The use of spermatozoa regardless of their motility for IVF may provide an approach for rescuing genetic materials from animals that died or underwent a vasectomy because of medical reasons. If the spermatozoa motility is not necessary for completing fertilization in ICSI assisted fertilization, it may be possible to use frozen-thawed spermatozoa without cryoprotectant to fertilize oocytes.
In the original study of ICSI in mammalian oocytes, Uehara and Yanagimachi (1976) showed that human spermatozoa frozen in isotonic solution without cryoprotectant can decondense and form pronuclei when injected into hamster oocyte, indicating that the spermatozoa nuclei is an extremely stable organelle. Furthermore, the genetic integrity of mouse spermatozoa and isolated spermatozoa heads was shown to be maintained after freezing and thawing in media containing glycerol. Normal offsprings were obtained after transfer of mouse oocytes fertilized by ICSI with such spermatozoa or spermatozoa heads (Kuretake et al., 1996). Normal mouse offsprings have also been obtained from oocytes microinjected with spermatozoa frozen in the absence of cryoprotectant (Wakayama et al., 1998).
In the present study we examined the ability of rat cauda epididymal spermatozoa cryopreserved without cryoprotectant in liquid nitrogen to induce pronucleus formation.
MATERIALS AND METHODS
Cryopreservation of Cauda Epididymal Spermatozoa
Cauda epididymal spermatozoa were isolated from adult (four to eight months old) Wistar rats. A small part of cauda epididymides tract was excised with a fine scissors and a small drop of spermatozoa mass was placed in one milliliter milli-Q water in a 1.5-ml tapered centrifuge tube and vortexing for about 30 seconds and each 100-l aliquot was transferred to another 1.5-ml tapered centrifuge tube. The swim-up method was performed to get the motile spermatozoa for 10 minutes in 37oC. The semen was then diluted using Hepes-modified Krebs-Ringer-Bicarbonate (Hepes-mKRB ) medium to reach the concentration of 200 x 106 cell/ml prior to loading in 0.5 ml straw. The straw was then heated-sealed and directly plunged into liquid nitrogen (-196°C) and stored in this temperature for up to one month.
Preparation of Oocytes
Adult (eight to 12 weeks old) female Wistar rats were induced to superovulate by i.p. injections of 25-30 IU eCG between 19:50 and 20:00 PM on the day of estrus and 25-30 IU hCG 48 hours later. The females were killed 13 hours after hCG injection and the excised oviducts were placed in a small drop (100-200 l) of Hepes-mKRB supplemented with 0.1% (w/v) hyaluronidase from bovine testes (Sigma) in a polystyrene culture dish (35 x 10 mm; Falcon No. 1008, Becton Dickinson Labware, Lincoln Park, NJ) at 37C. Oocytes with cumulus cells were released from the ampullar portion of the oviducts and kept in the medium for about five minutes. The cumulus-free oocytes were washed three times in Hepes-free mKRB containing 25.07 mM of NaHCO3, placed into 100 l of the same medium, and kept in a CO2 incubator (5% CO2 in air at 37C) for an hour until spermatozoa were microinjected.
Preparation of Spermatozoa Heads
The straw containing spermatozoa were taken out from liquid nitrogen container and placed in water at 37C for about one minute for thawing. Semen was then released from straw and placed into tube for performing sonication (ten 0.3 sec bursts at 0.7 sec interval) using a 20% power output of a Branson Sonifier Model 250 (Branson Ultrasonies, Danbury, CT) for three seconds. Separation into heads and tails was successful in more than 80%. A portion (100 l) of the sonicated suspension was transferred into another centrifuge tube containing one milliliter Hepes-mKRB and the diluted spermatozoa suspension was centrifuged at 800 g for three minutes. The pelleted material consisting of spermatozoa heads and flagella was resuspended in one milliliter Hepes-mKRB.
Microinjection of Spermatozoa Heads
The oocytes which had been kept in a CO2 incubator were initially transferred into 100-200 l mKRB-Hepes containing 5 g/ml cytochalasin B to prevent spontaneous activation which occurs in rat oocytes at a high rate (Keefer and Schuetz, 1982). Then five microliters medium containing 8-10 oocytes was placed in a cover of a petri dish (50 x 4 mm; Falcon No. 1006, Becton Dickinson Labware, Lincoln Park, NJ) and covered with paraffin oil (Nacalai Tesque Inc., Kyoto, Japan). A small drop (one microliter) of the suspension containing 20-40 spermatozoa heads and some tails was introduced into a drop containing oocytes. Microinjection of spermatozoa head into oocyte was performed at 37C using a piezo-driven pipette that was prepared from Borosilicate glass capillary tubes (Sutter Instrument Co., Novato, CA). The external diameter of the pipette tip was 4-5 m. A spermatozoa head was aspirated into the injection pipette so that the apex of spermatozoa head was positioned facing the opening of the pipette. The pipette tip was brought in contact with the zona pellucida of the oocyte which was held by a holding pipette. The external diameter of holding pipette was 100-110 m while its internal opening diameter was 15-20 m. The zona was drilled by applying a few piezo pulses. The pipette tip was then introduced into the perivitelline space and forced slightly into the oolemma prior to application of a few piezo pulses in order to puncture the oolemma. The spermatozoa head, in a minimal amount of medium that did dot contain polyvinylpyrrolidone (PVP) was expelled into the ooplasm. The injection of spermatozoa head into oocyte was completed within one hour after sonication of spermatozoa.
Determination of Oocyte Activation after ICSI
Spermatozoa heads isolated from spermatozoa were injected into oocytes. The injected oocytes were washed four times with Hepes-free mKRB containing 25.07 mM NaHCO3. Oocytes were then transferred into 100 l of the same medium covered with paraffin oil in a culture dish (35 x 10 mm; Falcon No. 1008) and cultured in a CO2 incubator (5% CO2 in air at 37C). At 10 hours after the start of culture, with morphologically normal oocytes were scored and grouped as ICSI-successed oocytes, while the rest were grouped as ICSI-failed oocytes. These ICSI-successed oocytes were then mounted, fixed with 2.5% (v/v) glutaraldehyde in phosphate buffer (pH 7.4) followed by 10% (v/v) neutral formalin and stained with 0.25% (w/v) lacmoid in 45% (v/v) acetic acid (Toyoda and Chang, 1974). The stained oocytes were examined for signs of oocyte activation and morphological changes of the penetrated spermatozoa head under a phase-contrast microscope. The activated oocytes showed different appearances based on the formation both of female and male pronucleus or changes in spermatozoa morphology determined by decondensation of spermatozoa nuclei. Whereas unactivated oocytes were indicated by the present of spermatozoa head without any changes (condensed sperm head) and without female pronucleus formation.
Statistical analysis
All data were provided in mean and were analyzed for significant differences between treatments using X2-test.
RESULTS AND DISCUSSION
As shown in Table 1, when oocytes were injected with frozen-thawed spermatozoa heads 45% oocytes were success ICSI and 55% oocytes were fail ICSI. The results were significantly (P
It has been shown that bovine spermatozoa remain alive and are functional even after cryopreservation in LN2 for up to 37 years (Leibo et al., 1994). In the mouse, successful cryopreservation of spermatozoa using raffinose plus skim milk (Okuyama et al., 1990) or glycerol (Tada et al., 1990) has been reported. Nakatsukasa et al. (2001) have recently obtained live rat offspring after intrauterine insemination with epididymal spermatozoa cryopreserved at -196C with various concentrations of glycerol. In the rat, however, IVF using cryopreserved spermatozoa is extremely difficult. Using the technique of ICSI, it is demonstrated that spermatozoa that lost their motility after freezing and even only spermatozoa heads isolated from spermatozoa can contribute to normal fertilization, when they were microinjected into oocytes, leading to development to offspring (Kuretake et al., 1996; Wakayama et al., 1998). These results indicate that spermatozoa nuclei are extremely stable and their genetic integrity is completely maintained under freezing temperatures.
Table 1. Successful ICSI using fresh and frozen-thawed spermatozoa head in rat oocytes
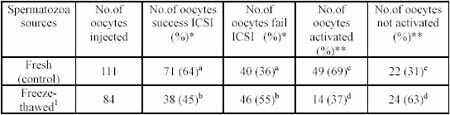
1Cauda epididimal spermatozoa stored for up to one month.
* Percentage of oocytes injected.
** Percentage of success ICSI.
CS = condensed spermatozoa
DS = decondensed spermatozoa
2PN = two pronucleus
a&b significantly difference at P
c&d significantly difference at P
Table 2. Rat oocytes activation after ICSI using fresh and frozen-thawed spermatozoa head
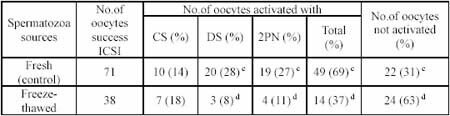
In the present study, we demonstrated that rat cauda epididymal spermatozoa cryopreserved in liquid nitrogen without cryoprotectant was able to activate oocytes to form female pronuclei (37%). Moreover, the interaction between oocytes and spermatozoa prior to the unity of both pronucleus were present in considerable number (19%) indicated by decondensation of spermatozoa head and formation of two pronucleus. Parrington et al. (1996) reported that chemical substance carried by spermatozoa which promoted activation in oocytes is called sperm-borne oocytes activating factor (SOAF). This protein which is then called oscillin have 33 kDa molecular weight and located in equatorial segment of spermatozoa acrosome. Oocytes activation may cause resumption of second meiosis indicated by the extrusion of second polar body and formation of female pronuclei. Subsequently, intracellular glutathione (GSH) mobilization and cytoplasmic proteins activation occured simultaneously in which GSH may cause decondensation of spermatozoa, a prerequisite event of male pronucleus formation, and one of the cytoplasmic protein called tubulin may play a role in the unity of male and female pronucleus. Perreault et al. (1988) stated that in order that spermatozoa DNA can participate in embryonic development, it must be unpackaged in the oocyte early in the process of fertilization. An initial event of the unpackaging process is a reduction in the protamine disulfide bonds that have been formed during epididymal maturation. The results explained that the nuclei of spermatozoa are fairly resistant even exposed to liquid nitrogen without cryoprotectant. Yanagida et al. (1991) examined the thermostability of mammalian spermatozoa nuclei and reported that mammalian spermatozoa nuclei do not loss their ability to form pronuclei or to synthesize DNA even when exposed to high temperature (90C for 30 minutes).
Figure 1. Different stages of rat oocytes development following Intracytoplasmic Sperm Injection.
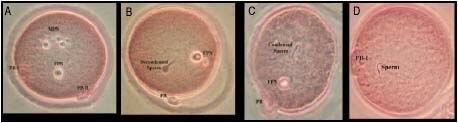
A= Oocyte with male and female pronucleus
B= Oocyte with female pronucleus and decondensed sperm
C= Oocyte with female pronucleus and condensed sperm
D= Oocyte without any pronucleus and condensed sperm
CONCLUSION
In conclusion, the present study indicates that separated spermatozoa head resulted from frozen-thawed rat cauda epididymal spermatozoa cryopreserved without cryoprectants was able to induce pronucleus formation.
REFERENCES
Keefer CL and A.W. Schuetz. 1982. Spontaneous activation of ovulated rat oocytes during in vitro culture. J Exp Zool., 224:371-377.
Kuretake, S., Y. Kimura, K. Hoshi and R. Yanagimachi. 1996. Fertilization and development of mouse oocytes injected with isolated sperm heads. Biol Reprod, 55:789-795.
Leibo, S.P., E. Semple and T.G. Kroetsch. 1994. In vitro fertilization of oocytes by 37-year-old cryopreserved bovine spermatozoa. Theriogenology, 42:1257-1262.
Nakatsukasa, E., T. Inomata, T. Ikeda, M. Shino and N. Kashiwazaki. 200l. Generation of live offspring by intrauterine insemination with epididymal spermatozoa cryopreserved at –196 degrees C. Reproduction, 122(3):463-467.
Okuyama, M., S. Isogai, M. Saga, H. Hamada and S. Ogawa. 1990. In vitro fertilization and artificial insemination by cryopreserved spermatozoa in the mouse. J Fertil Implant, 7:116-119.
Parrington, J., K. Swann, V.I. Schevchenko, A.K. essay and F.A. Lai. 1996. Calcium oscillation in mammalian eggs triggered by a soluble sperm protein. Nature, 379:364-368.
Perreault, S.D., R.R Barbee and V. Slott. 1988. Importance of glutathione in the acquisition and maintenance of sperm nuclear decondensing ability in maturing hamster oocytes. Dev Biol., 125:181-186.
Tada, N., M. Sato, J. Yamanoi, T. Mizorogi, K. Kasai and S. Ogawa. 1990. Cryopreservation of mouse spermatozoa in the presence of raffinose and glycerol. J Reprod Fertil., 89:511-516.
Toyoda, Y., and M.C. Chang. 1974. Fertilization of rat eggs in vitro by epididymal spermatozoa and the development of eggs following transfer. J Reprod Fertil 36:9-22.
Uehara, T and R. Yanagimachi. 1976. Microsurgical injection of spermatozoa into hamster eggs with subsequent transformation of sperm nuclei into male pronuclei. Biol Reprod., 15:467-470.
Wakayama, T., D.G. Whittingham. and R. Yanagimachi. 1998. Production of normal offspring from mouse oocytes injected with spermatozoa cryopreserved with or without cryoprotection. J Reprod Fertil., 112:11-17.
Wildt, D.E., S.L. Monfort, A.M. Donoghue, L.A. Johnson and J. Howard. 1992. Embryogenesis in conservation biology or how to make an endangered species embryo. Theriogenology, 37:161-184.
Yanagida, K., R. Yanagimachi, S.D. Perreault and R.G. Kleinfeld. 1991. Thermostability of sperm nuclei assessed by microinjection into hamster oocytes. Biol Reprod., 44:440-447.
September 21st, 2006 at 10:22 pm
apakah pernah dilaporkan kejadian O157:H7 pada ayam?apakah ada pustakanya yang dapat membuktikannya?